Elucidating the Structure of Tomato Enzymes (Similar to Natural Rubber Synthases) ~Joint Research with Tohoku University, Kanazawa University & RIKEN~
Mar. 03. 2022
Sumitomo Rubber Industries, Ltd. is pleased to announce that, through joint research undertaken with Associate Professor Seiji Takahashi of Tohoku University, Associate Professor Satoshi Yamashita of Kanazawa University and Group Director Masaki Yamamoto, Kohei Takeshita and other researchers at the RIKEN SPring-8 Center, we have elucidated the structure and succeeded in functional modification of cis-prenyltransferase (NDPS1)*1, an enzyme that is found in tomatoes and that has a similar structure to the enzymes in the biosynthesis of natural rubber. This research was made possible thanks to the utilization of the cutting-edge SPring-8*2 Synchrotron Radiation Facility.
We are also pleased to announce that the results of this research were published in the February 8 issue of The FEBS Journal, which is a leading international journal in the field of life sciences.
 |
 |
|
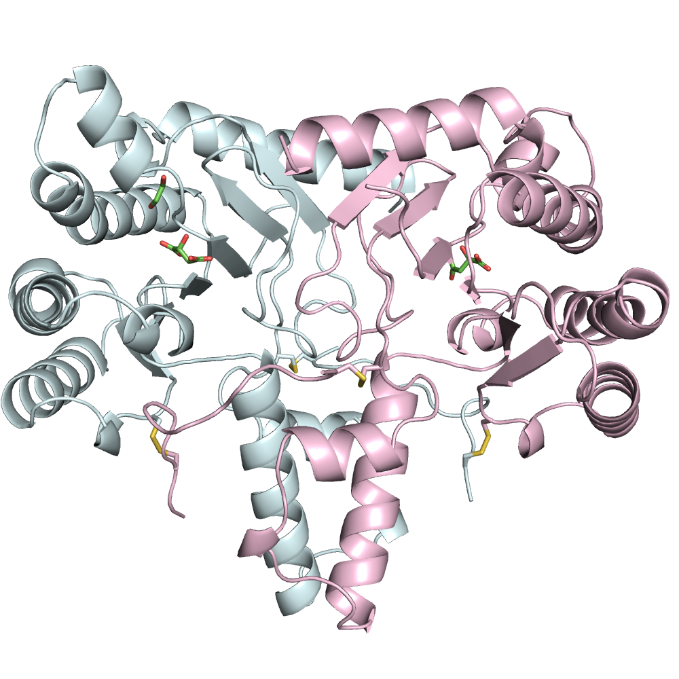
Ribbon Model of NDPS1 from Tomato:Homodimer Formed by Bonding of 2 Enzymes of the Same Type (Light Blue & Pink) |
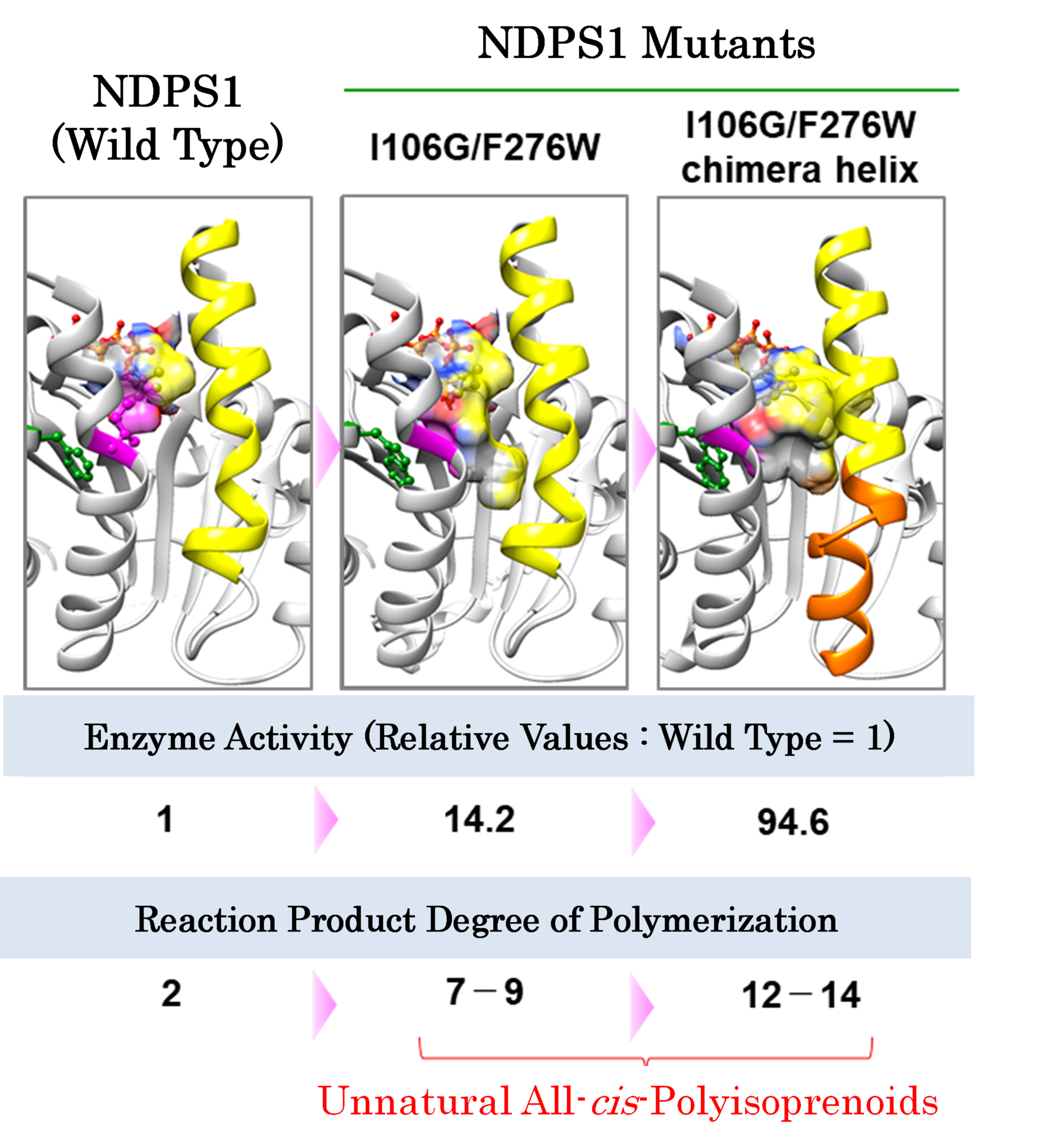
NDPS1 Mutant Structural Models & Properties Comparison |
NDPS1 is an enzyme belonging to the cis-prenyltransferase family, which also includes the enzymes involved in the biosynthesis of natural rubber. As such, it has long been presumed that the two would likely have similar structures. As the properties of NDPS1 make it better suited to structural analysis than the natural rubber synthases found in Pará rubber trees, our joint research team set about studying the structure of NDPS1 using SPring-8. As a result of our research, we have not only shed light on the structure of NDPS1 and determined the key sections affecting the length of its synthesis products, but have also succeeded in increasing the degree of polymerization and significantly enhancing the chemical reactivity of synthesis products by introducing new mutations to these key sections. With this breakthrough, it has now become possible to biosynthesize compounds that do not exist in nature.
It is our hope that elucidating the structure of NDPS1 will make it possible to shed further light on the mechanisms behind the biosynthesis of natural rubber, which in turn will allow for new advancements in the development of technologies to ensure a stable and reliable supply of natural rubber.
<The FEBS Journal Official URL> https://doi.org/10.1111/febs.16392
※1. NDPS1 is an enzyme involved in the formation of terpenes (isoprene compounds that serve as the precursors for many useful substances found in plants) as well as the biosynthesis of natural rubber.
※2. SPring-8 is the world’s most powerful synchrotron radiation facility. (Location: Sayo, Hyogo, Japan)
<Reference>
■Previous News Release (Issued on October 26, 2016)

